Ultrafast THZ Optoelectronic Laboratory
Far Infrared, THz Time Domain Spectroscopy of Flames
For commercial as well as scientific reasons, combustion processes have been the subject of intensive investigation. Important questions for understanding and accurately modeling combustion are what species are present in the flame, and the spatial distribution and temperature of these species. A wide variety of laser based techniques have been used to measure one or more of these parameters such as laser induced fluorescence, four wave mixing, and various Raman processes. All these techniques have some advantages and disadvantages, but since no method is capable of providing a complete characterization of flames, alternative techniques are often used in conjunction.
Using the technique of THz Time Domain Spectroscopy (make this a hyper text link) we have made the first panoramic far infrared (THz) absorption measurements of combustion products in a premixed laminar hydrocarbon-air flame. The comprehensive spectra we obtain provide a survey of the relative abundance of the constituents of the flame. Such overview data is valuable in guiding higher resolution methods to spectral regions of interest and in determining absolute concentrations of important constituents for application of other techniques. Using THz-TDS we also determine the flame temperature by comparing relative populations of rotational states of water vapor. Prior to these experiments, comprehensive far-infrared (THz) absorption spectroscopy of flames has not been possible.
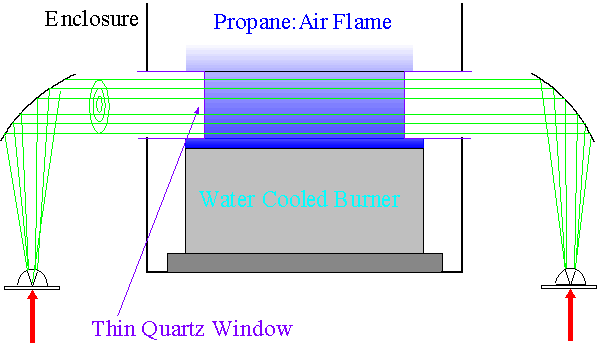
As depicted in the figure below, the
The atmospheric pressure flame is stabilized on a water cooled, porous sintered bronze burner so the spatial geometry and composition remain constant in time. We used a stoichiometric propane:air flame for these experiments. By using the technique of THz Time Domain Spectroscopy we can analyze the components of the flame.
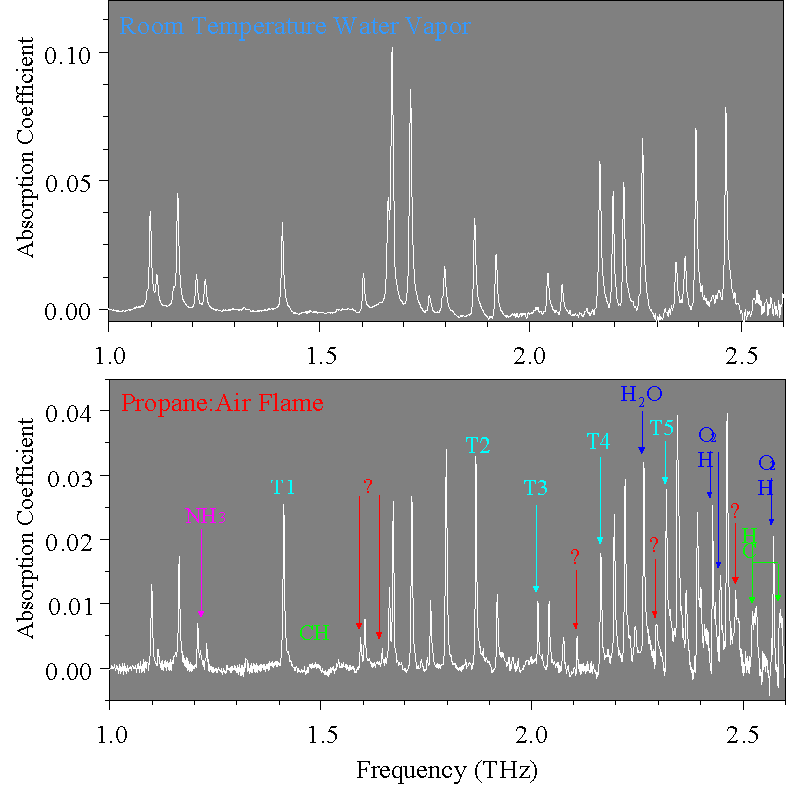
Absorption spectra of the laboratory air data and a propane:air flame are shown in
the figure below.
H2O (blue)
CH (green)
NH3 (pink)
? (red)
All the absorption lines in laboratory air are due to water vapor. Also, since the main combustion products of propane are carbon dioxide (invisible to our measurement) and water, most of the absorption lines of the flame are due to the generated water vapor. All of the lines of the top figure appear in the flame spectrum, but with several differences: the relative heights have changed, the lines have narrowed, several new lines have appeared, and the total absorbance has dropped. Most of these changes are directly attributable to thermal effects within the flame- for example, the new "H2O" lines in the lower figure are from high energy rotational states of water.
Several of the new absorption lines are tentatively identified with the species CH and NH3 by a comparison with tabulated line strength data from a web site at Jet Propulsion Laboratories. Given the relative absorption of H2O, CH and NH3 , the flame temperature of 1150 K,and a 1.2 GHz line width, we estimate that at the sampled position the flame contains 3.3% water, 0.4 % CH, and 0.8% NH3. Five unidentified absorption lines (?) are visible as well. We suspect that some of these lines may be due to methylene CH2 free radicals, but we have not been able to verify this due to an absence in the literature of their comprehensive far-infrared spectra. This situation illustrates the potential importance of THz-TDS for transient species.
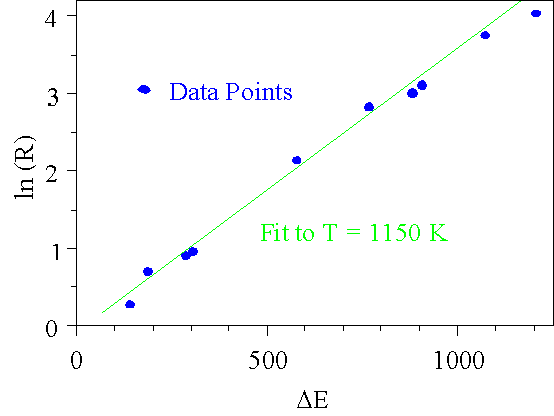
Given that the relative populations of the rotational states are determined by Boltzmann statistics, the temperature of the flame may be obtained from the relative change between absorption lines of known energy.
By taking the ratio of two absorption lines, RAB(T) = aA/aB, at an unknown temperature T and the same ratio RAB(To) at a known temperature To, the ratio RAB(T)/RAB(To) is used to determine the temperature by logarithmically plotting the measured ratio RAB(To)/RAB(T) versus EB'-EA'. This is shown in the figure below.
The temperature T = 1150 80 K is determined by the slope of the fit and is in excellent agreement with the temperature T = 1150 40 K measured using a thermocouple under identical propane:air mixtures 1 cm above the burner surface.
If you would like to learn about this in more detail see our list of publications.